CCD BIOES SANTE
a global support for the
distribution and promotion of pharmaceutical products
Boost your expansion
At CCD BIOES SANTE, we are committed to supporting you at every stage of your pharmaceutical and para-pharmaceutical products’ distribution and promotion.
We offer our expertise to effectively promote your products in the market, ensuring the success of your launches. Our expert team works closely with you and your brands and nurtures them among healthcare professionals.
At CCD BIOES SANTE, we firmly commit to a policy in favor of the quality of promotional information, ensuring an ethical and transparent approach in all our actions.
Furthermore, we provide personalized advice and manufacture products in all forms, tailored to your specific needs, to ensure your success in the pharmaceutical market.
The monitoring of your products from A to Z
Entrust your products to us; we ensure comprehensive monitoring from A to Z, enabling accelerated distribution and promotion in the pharmaceutical sector.

Promoting your pharmaceutical and para-pharmaceutical products
From face-to-face promotion to sophisticated digital strategies, including key performance indicators management (KPIs) and field effectiveness (SFE), we cover all aspects of promotion.
Our dedicated team also handles customer service, key account management, the establishment of high-performing call centers, and tailored training programs to meet your specific needs.

The success of your product launches
From preparing ACL sheets and necessary regulatory filings to diligently tracking regulatory key performance indicators (KPIs) and medical information, and overseeing vigilance and complaint management, we ensure mastery of every step to ensure the successful deployment of your new products in the market.
Incorporating your brands to expand their reach among healthcare professionals
Brand Management: Marketing Strategy, Business Planning…
BUY & SELL: Distribution, P&L Management, Promotion, Communication…
SUPPLY CHAIN: Procurement, Inventory Management, Reporting…

Promoting your products to the medical community
Target audience : Gynecologists, Midwives with private practice in urban and hospital settings.
CCD BIOES SANTE is committed to a policy of quality regarding promotional information.
CCD BIOES SANTE is certified for its information activity through canvassing or prospecting aimed at promoting medications by an independent organization following the certification standards of the French National Authority for Health (HAS) from March 2017.

Committed to a policy in favor of the quality of promotional information
Several stages mark the life of a healthcare product, from its development to its availability in hospitals, clinics, and pharmacies.
Each stage follows regulation affairs requirements – French, European, and international – to which CCD BIOES adds its commitment to ethical action.
Consultation and manufacturing of products in all forms
Bailly Creat is a French pharmaceutical manufacturer specializing in dry-form medication production.
Prodimed is a leading French player in the design and manufacturing of aesthetic medical devices.
LABOCREATION France, isour laboratory developing and producing “Made in France” cosmetics.
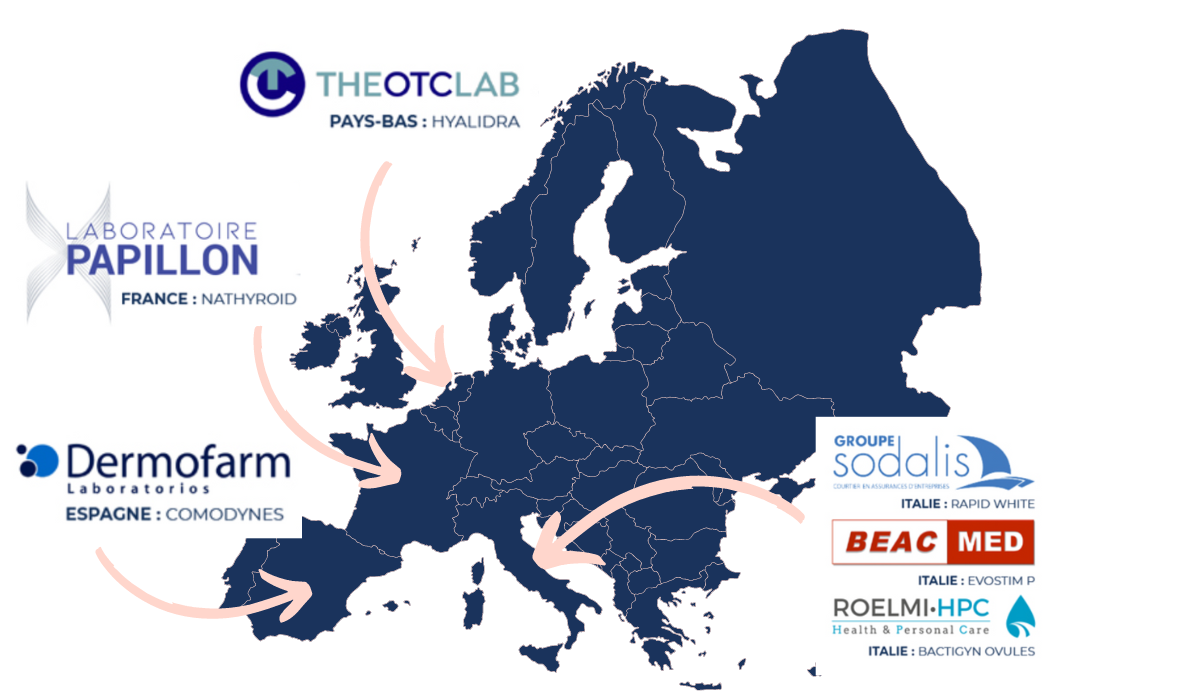
Partnerships across Europe
We have forged partnerships across Europe, strengthening our presence and expertise in the pharmaceutical sector. These strategic collaborations enable us to offer our clients tailor-made solutions adapted to the specificities of each European market while ensuring efficient distribution and successful promotion of their products.
Ethical communication
CCD BIOES SANTE is committed to the ethical guidelines outlined by the Charter for Information provided for the promotion of medicines and its reference framework through prospecting and canvassing. Your representative remains available to present these rules and address your questions.
CCD BIOES SANTE is certified for its information activity through canvassing or prospecting aimed at promoting medications by the independent organization AB certification, by the certification standards of the French National Authority for Health (HAS) of March 2017.
Additionally, Laboratory Horus Pharma has committed to a quality policy regarding promotional information.
They have chosen us
as their trusted partner.
















